Innovation Is The Core Of The 4Pack Ethos
We pride ourselves on being different from the rest, revolutionising the market by combining product and packaging specification with artwork management for various industries. What sets us apart is our dedication to providing tailored solutions that cater to the unique challenges of each client we serve, whether it’s food, beverage, consumer goods, wine, or retail.
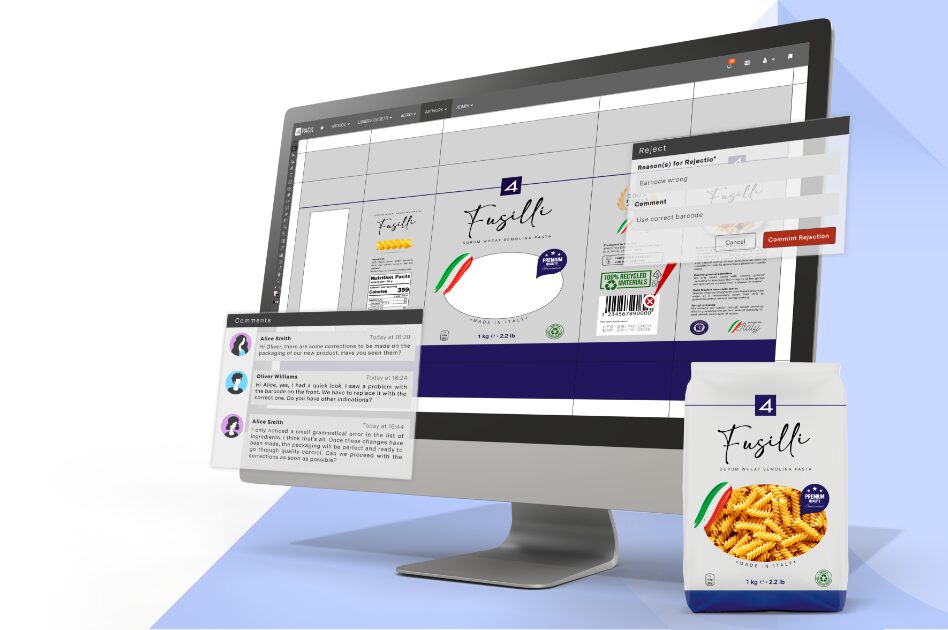
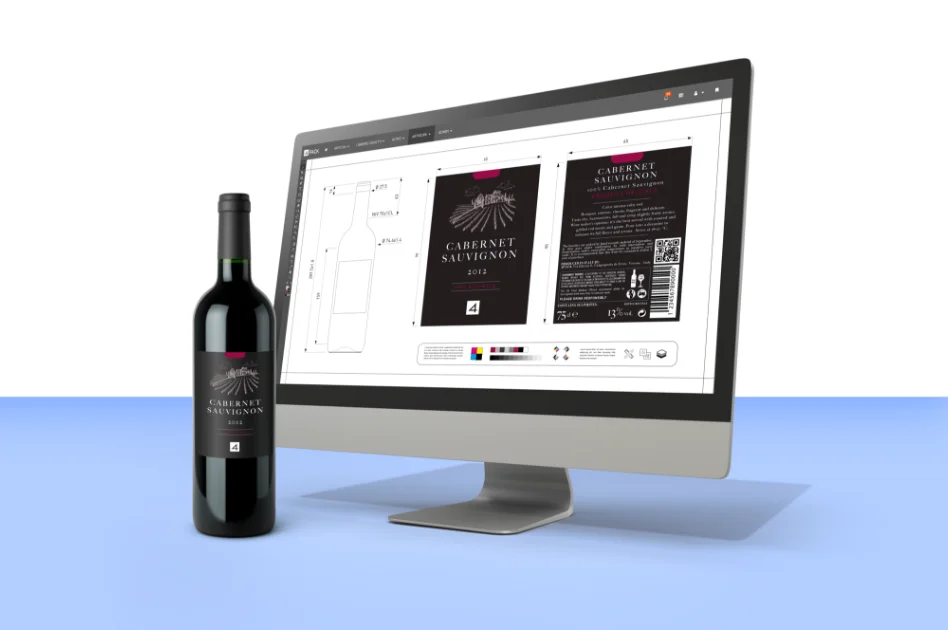
Our platform seamlessly integrates product and packaging specification data and artwork with streamlined workflows to enhance efficiency. With our advanced artwork management tools, you can ensure compliance, accuracy, and brand governance, driving your products forward.
With years of experience in the product, packaging and artwork management domain, our team of industry experts brings unparalleled expertise to every project. We understand the intricacies of the industries we serve, allowing us to fine-tune our solutions to address the daily challenges faced by businesses.
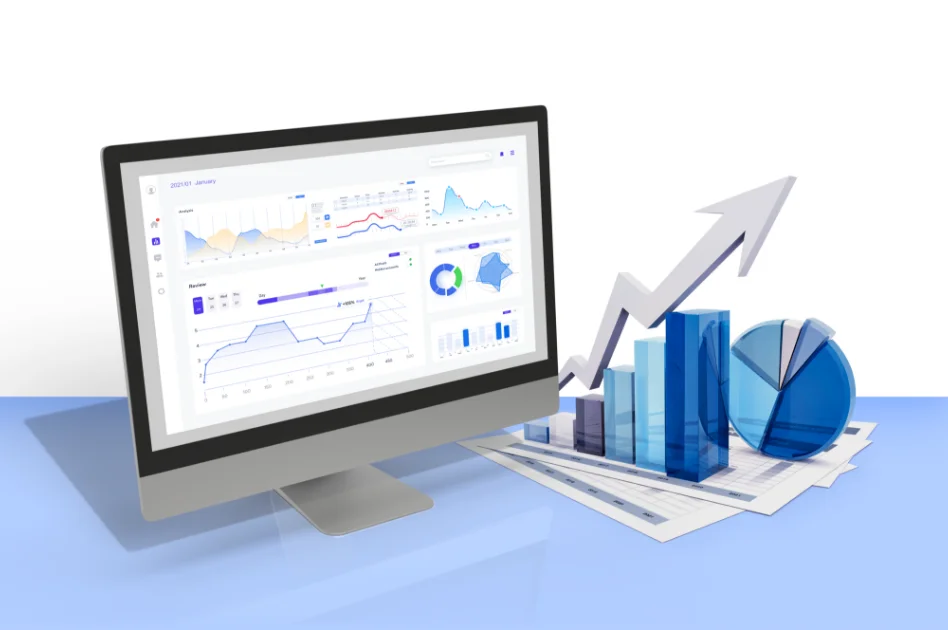
At 4Pack, we focus on delivering instant results, recognising the fast-paced and competitive nature of the consumer goods market. Our tools empower you to make quick, informed decisions, giving you a competitive edge.
Innovation is at the core of our ethos, as we continually invest in research and development to stay ahead of industry trends.
Our customer-centric approach is fundamental to everything we do. We take the time to understand your unique requirements and challenges, and our dedicated support team is always ready to assist you every step of the way.